How many of us started today with a tea or coffee? When you boiled the kettle, how hot was it? It sounds like a stupid question – 100°C is boiling. But how do we know that is the case? In fact, because I am at sea level, my water will boil at 100°C, so the steam coming out of my kettle will also be 100°C. It is 100°C at sea level, which is 0bar g, about 1bar abs.
If I pour the kettle at the top of Mount Everest, there would be a lower pressure because there is less air above it pushing down, so my water boil at 70°C. Let’s say I went to the lowest place on earth, which is actually the dead sea in Jordan, and I wanted to brew up. How hot would my boiling water be? The answer is 101.5°C.
In our steam system, I want to focus on what happens when the pressure rises. A graph of the steam saturation curve shows that as you increase pressure, the boiling temperature rises.
Our dry saturated steam tables, which are available as an app, go into more detail. Looking at just the first row, pressure at sea level is 0bar g, and the boiling temperature is 100°C. The next three columns to the right are about the heat energy in the different phases of water. This is measured per kg water: one litre. The amount of energy in 1kg of water in these conditions is 419kJ energy. The next column, hfg, is the amount of energy that I need to evaporate that 1kg water at 100°C into steam: 2,257kJ. Now if we turn that whole kilogramme of water into steam, total amount of energy is hg. That’s just hf plus hfg: 2,676kJ. You can see from this that there is more than six times as much energy in 1kg of steam than is in 100°C water.
Let’s say that steam condenses on our mirror. How hot would the condensate be on the mirror? Initially, it’s just gone through phase change, back to hf, so it’d be 100°C, and still have 419kJ of energy: hf from my table. Remembering that the total amount of energy in the steam was 2,676kJ of energy, I have 419 left in condensate on the mirror, so how much energy has gone into mirror? It’s hfg: 2,257kJ energy into mirror. That’s all usable energy, even if we don’t talk about the energy in the condensate.
The last column is the volume that that amount of water will take up. For 1kg of water, it was 1l, or 0.001m3. When I turn it into steam, it has got 1,673 times bigger; the size of a small skip. The pipes that we would need to move that around our factory would need to be really big and really expensive. You’d have to have more lagging, there would be more surface area in the pipe for the steam to heat up, and all of the fittings would be really expensive because they would be pipe-sized.
When we pressurise our steam to 2bar g, the first thing to notice is that our boiling point has gone up; it is now 134°C. And hf has gone up and so has hg. But wait a minute – hfg, usable energy, has gone down! And volume has gone down by more than half. So now we can use smaller pipes to transport it than we would at zero bar gauge.
You can see from the table that those trends carry on at higher pressures. From this we can say from this that there is more energy in lower-pressure steam than higher.
On the other hand, I want to run my boiler at maximum design pressure; that’s when it’s operating at its best efficiency. And because the steam is under pressure, it takes up less room; we need smaller pipes, ancillaries, and require less lagging. So we want to distribute at pressure.
But. Because there’s more energy at lower-pressure steam – 163 units more at 2 bar g compared to 10bar g – we want to reduce pressure at point of use. If you were to plot volume versus pressure, you would see that as pressure rises, its volume drops. This is really important in our boiler, because if we have a big bubble of steam that comes to the top of our boiler water, and it bursts, it will flick loads of water all over the place. The bigger the bubble, the bigger the flick. If the same thing happens at 10bar g, the table tells us that the bubble is 10 times smaller. That means that as it bursts, there will be less water flicked about. Which means our steam will be much drier.
We want the steam to be as dry as possible. First, we saw from the steam tables that it’s the steam, not the water, that carries the masses of energy. Secondly, wet steam will cause erosion then corrosion of our pipework and ancilliaries. It goes without saying that we want the steam as clean as possible; because if not it will clog up control surfaces and cause steam traps to fail.
What else do we want? First, from a safety point of view, we have to make sure that we only work to our risk assessment or standard operating procedures; we want to get our steam there as safely as we can. Next, we want the right quantity of steam; otherwise, our plant could suffer from steam starvation. And as we’ve seen on the steam tables, steam pressure is directly linked to temperature. So we want the right pressure hence temperature at our point of use.

Imagine I have a cooking vessel. The colour is green, so let’s say I’m cooking pea and mint soup. Our boiler is running at 10bar g, which means that the steam temperature is 184°C, which means that my soup will burn. As I reduce the pressure, the temperature of my steam will decrease. I’m going to reduce my pressure with a pressure reducing valve – the one I’ve got on there is a direct acting pressure reducing valve – to 1bar g. Now I have a steam temperature of 120°C and my soup will cook nicely without burning.
We might have to drop that pressure less, it just depends on the product or process that we’re doing. For instance, If I were doing sterilisation, I would want my steam to be about 134°C, which equates to about 2.2bar g.
Why else might I want to reduce pressure? What if the cooking vessel isn’t rated to 10bar g that my boiler’s running at. (As a pressure vessel, it should have a nameplate on it with all of the details, such as safe working pressure, normal working pressure, maximum accumulated pressure. This will also be included on the document called the written scheme of examination that sits between you and your insurer). Let’s just imagine that this cooking vessel can’t see any more than 6bar g steam. Its normal working pressure is 1bar g. What would happen if my pressure reducing valve failed and we have more than 6bar g coming down the line? Don’t panic; there is a safety valve there to save the day. That will also be on the written scheme of examination. Depending on your insurance company, it might be inspected every six months or year. The inspector will want to see it all apart and that it will lift at its set pressure.
We’ve reduced the pressure before the pressure reducing valve. It was 10bar g, now it’s 1bar g. The condensate going into my steam trap is at 1bar g. A return line takes the condensate back to the boiler house, because it has all of that energy in it, and remember, water is valuable. It is at 0bar g. Whenever condensate goes from a higher pressure to a lower pressure, it will make some flash steam. The bigger the pressure drop, the more flash steam. From 10bar g to 0bar g, I will make much more flash steam than from going from 1bar g to 0bar g. By reducing the pressure, we have made much less flash steam, and so produce fewer losses.
Finally, at the pressure reducing valve itself, one side has 10bar g steam. It’s got all of that energy. I’m going to reduce it to 1bar steam, which has less energy. What happens when that energy is released? What actually happens is that it has a drying effect on the steam.
BOX: Spirax Sarco training
This article is an edited version of Spirax Sarco’s webinar ‘Why reduce pressure in a steam system?’ featuring Mike Maslanka and UK steam technology centre manager Sally O’Connell. It was originally broadcast in January 2021, but is available to watch again via www.is.gd/iwuvij, where other recorded webinars are also available to view.Spirax Sarco says: “Even with changes to people’s working environment there is still a need for training and upkeep of skills.“ See also brochure: www.is.gd/otuboy.
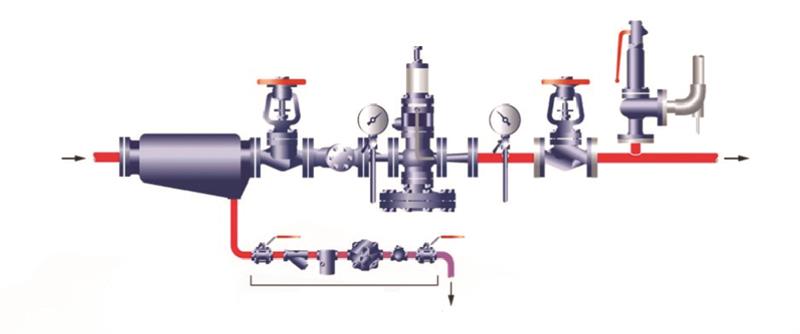
BOX: A typical pressure-reducing valve station
From left to right: A separator takes out water from the steam. Wet steam makes fine score marks in valve seats, eventually preventing it from closing. Then there is an isolation valve – a single-isolation valve is pictured here for simplicity, but I would use a double-block and bleed valve. To clean the steam is a strainer with 100-mesh screen to block debris. A pressure gauge confirms that the input pressure is as expected. The pressure-reducing valve depicted here is pipe-operated, which differs from the direct-acting mechanism used on the cooking vessel. Downstream of that is a second pressure gauge, to set the PRV to the desired output pressure. A second isolation provides two functions: for line maintenance and to create a no-load condition for set-up. Finally there is a safety valve to protect the plant from overpressure in case the PRV fails.-Mike Maslanka